强生Simponi获FDA批准用于溃疡性结肠炎好医师网
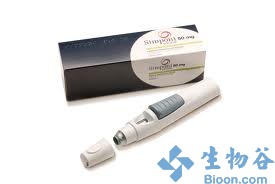
2013年5月16日讯 /生物谷BIOON/ --强生(JNJ)今天宣布,FDA已批准将Simponi( golimumab,好医师网,戈利木单抗)用于中度至重度溃疡性结肠炎(ulcerative colitis )患者的治疗。
Simponi为新一代全人源化的抗人抗肿瘤坏死因子α(TNF-α)单克隆抗体,能与可溶性和跨膜活性形式TNF-α结合,阻止其与TNF受体结合,从而抑制TNF的生物活性。
此前,FDA及加拿大卫生部已批准将Simponi与甲氨蝶呤(MTX)联用,来减少中度至重度活动期类风湿性关节炎(RA)、中度至重度活动期银屑病关节炎(PsA)、改善常规药物治疗无效的强直性脊柱炎(AS)成人患者的症状和体征。
类风湿性关节炎、银屑病、强直性脊柱炎、溃疡性结肠炎均属于自身免疫性疾病,这类疾病是由于自身免疫反应导致组织器官损伤和相应功能障碍。溃疡性结肠炎,其炎症能够导致结肠内壁溃疡,引发胃痛、胃肠道出血及腹泻。
在溃疡性结肠炎患者中开展的临床试验中,Simponi治疗组最常见的副作用为上呼吸道感染、注射位点红肿。(生物谷Bioon.com)
英文原文:FDA approves J&J's Simponi to treat ulcerative colitis
(Reuters) - The Food and Drug Administration has approved Johnson & Johnson's drug Simponi for patients with moderate to severe ulcerative colitis, an inflammatory disease affecting the colon.
Simponi is already approved to treat rheumatoid arthritis. Like RA, ulcerative colitis is an auto-immune disease in which the body's immune system attacks its own organs.
In the case of ulcerative colitis, inflammation can lead to open sores or ulcers in the lining of the colon, causing stomach pain, gastrointestinal bleeding and diarrhea.
The most common side effects of Simponi in clinical trials of patients with ulcerative colitis were upper respiratory infection and redness at the site in which the drug is injected.
Patients treated with Simponi, known also as golimumab, are at increased risk of developing serious infections, reactivation of Hepatitis B infection, heart failure and certain nervous system disorders.
The drug is marketed by J&J's Janssen Ortho Biotech unit.
(责任编辑:lili.zhao)
您还可以这样阅读