科学网科学网大学医学院 专家学术讲座:乳腺癌的最佳治疗方法好医师网

Dr. Shaw:
My name is Alice Shaw; I’m one of the thoracic oncologists at Massachusetts General Hospital in Boston. While crizotinib is very effective for patients with advanced ALK-positive lung cancer, unfortunately, by about a year, many patients will have signs of developing resistance. This is a huge barrier in terms of the benefit of crizotinib.
Today I’ll be talking about resistance to the ALK inhibitor crizotinib and some of the exciting new developments in overcoming crizotinib resistance; this focuses primarily on a whole class of new drugs that are called next-generation ALK TKIs [or tyrosine kinase inhibitors].
Dr. Shaw:
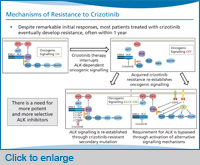
There’s been a lot of research into this question of crizotinib resistance [and] how it develops in patients. One of the well-established mechanisms of crizotinib resistance involves alterations in the ALK gene. And that can either be in the form of a new resistance mutation that occurs within the ALK tyrosine kinase domain, or it can be amplification of the ALK fusion gene—and because of either a mutation or amplification of the target gene, that confers resistance to crizotinib. This particular category of crizotinib resistance mechanisms has been very well worked out, and we see [these] resistance mutations or ALK fusion gene amplification in about one-third of our patients who have become resistant to crizotinib.
In the remaining two-thirds of patients, a subset of them have evidence of activation of alternative signalling pathways—we often call these bypass tracks because they allow the tumour cells to bypass inhibition of ALK and now to use other signalling pathways, for example, EGFR signalling or KIT signalling. And this is just beginning to be validated in patient samples.
Go online to view the complete activity
Dr. Shaw:
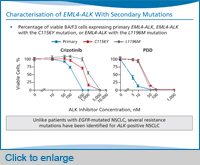
The first report of a patient with crizotinib resistance was a younger patient who was ALK-positive and had been on crizotinib and responded, but unfortunately relapsed after just only about five months. And at the time of relapse, they re-biopsied his resistant tumour, and they identified two resistance mutations.
The one that was most notable was the gatekeeper mutation in ALK, which is called L1196M, because it’s analogous to the T790M mutation in EGFR. The gatekeeper mutation is believed to confer resistance through steric interference of crizotinib binding to ALK [kinase]. These authors also found a second independent resistance mutation, C1156Y.
Since then, there have been a number of crizotinib-resistant patients identified that tell us that there are actually quite a few different resistance mutations beyond the gatekeeper [mutation] L1196M. This is really in contrast to what we see with EGFR-mutant lung cancer, where T790M is really the only resistance mutation that we identify in EGFR-resistant patients.
Dr. Shaw:
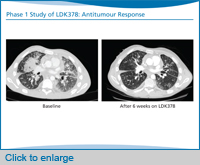
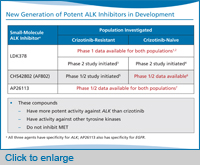
So with that background, we’ll move onto these new ALK inhibitors. This slide shows three ALK inhibitors that are the most advanced in clinical trials. This includes LDK378, which just opened phase 2 trials globally. The phase 1 trial of LDK378 has enrolled over 200 patients to date, so by far we have the most data on LDK378 compared to any of the other ALK inhibitors in development. That one is the one to keep an eye on, particularly in terms of registration trials, as well as potentially accelerated approval because the activity looks so promising in the crizotinib-refractory population.
There’s also a compound called CH542802 (we often just call it AF802) that’s currently in a phase 1/2 study, and then, there’s a compound, AP26113, that’s currently in a phase 1 study in the US.
What’s notable about all three of these compounds is that they are very selective for ALK, and they’re more potent against ALK than crizotinib. The other notable thing is that they inhibit ALK just like crizotinib, but they don’t inhibit MET. Crizotinib actually was first developed as an anti-MET drug, and it was only subsequently then developed to target ALK. None of these three drugs has any significant activity against MET, but they do have activity against other kinase targets.
Narrator:
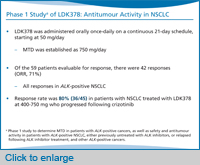
These potent ALK inhibitors have demonstrated preclinical activity that formed the rationale for recent and ongoing clinical trials, with LDK378 being furthest along in development. As of March 2013, the US FDA has granted LDK378 breakthrough drug status.
Dr. Shaw:
What’s notable immediately is that the response rate to LDK[378] is remarkably high in these patients—80% at doses of 400 mg or higher—the majority of who had become resistant to crizotinib, although we also do have some patients who are crizotinib-naïve. I should note that the phase 1 study of LDK378 has already established the maximum tolerated dose to be 750 mg. And this is the dose that has gone forward into the expansion phase of the phase 1 study, as well as the current phase 2 studies.